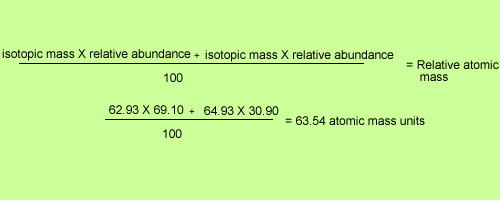
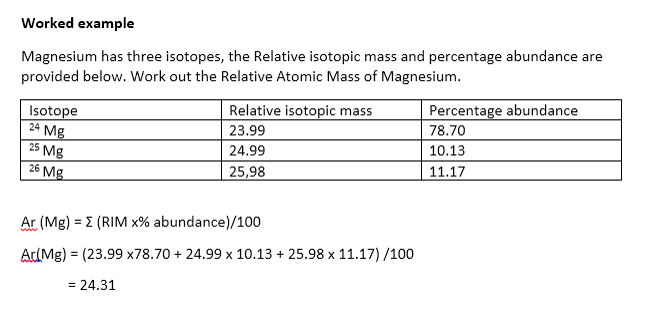
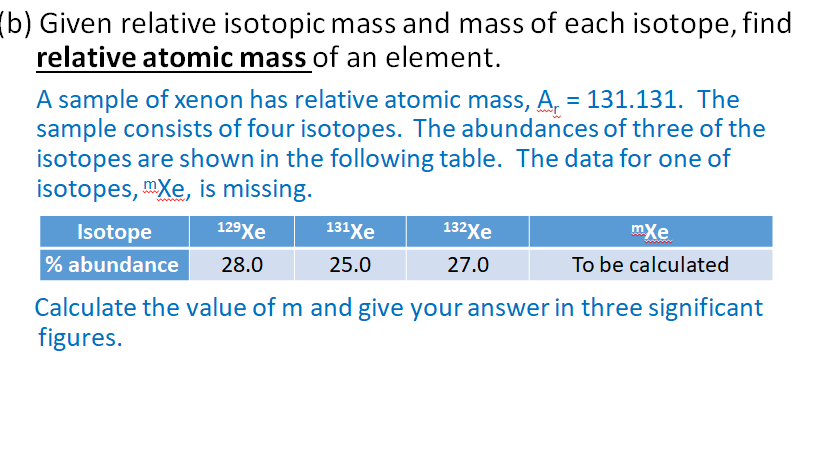
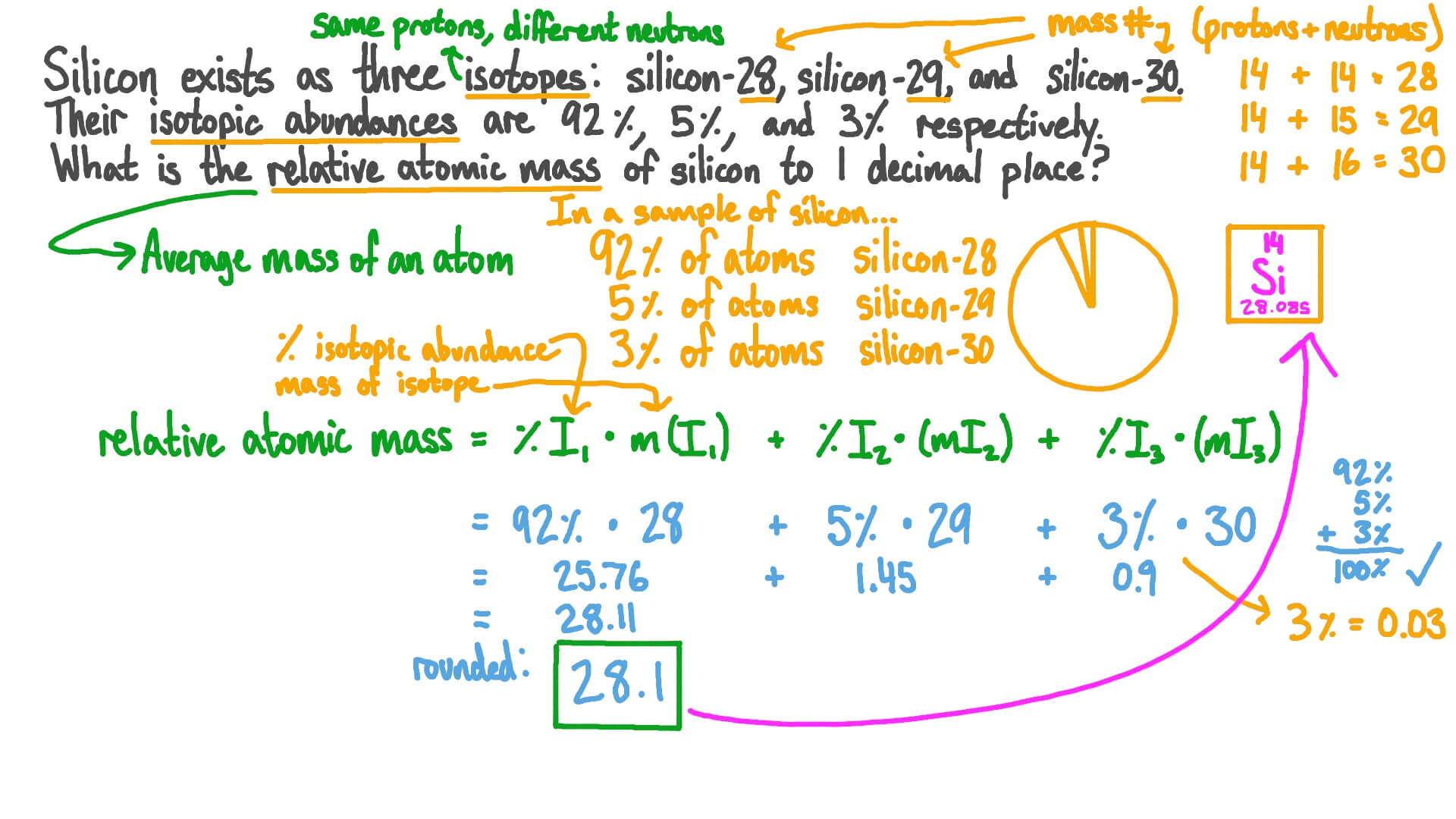
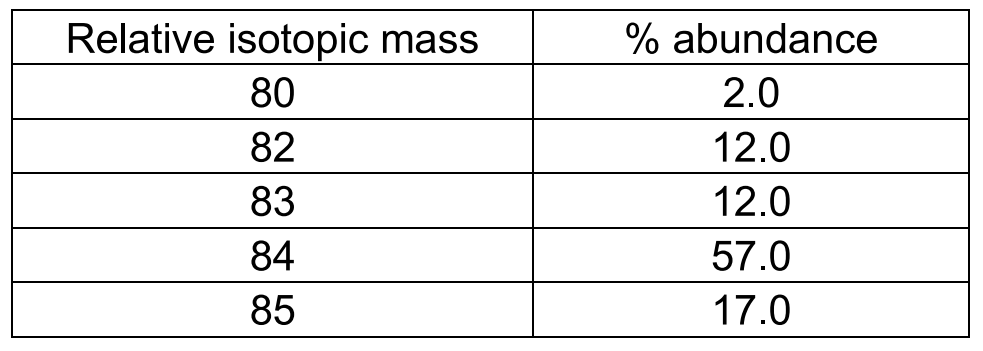Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes: IsotopeIsotopic molar mass Abundance ^36Ar 35.96755 g mol^-1 0.337

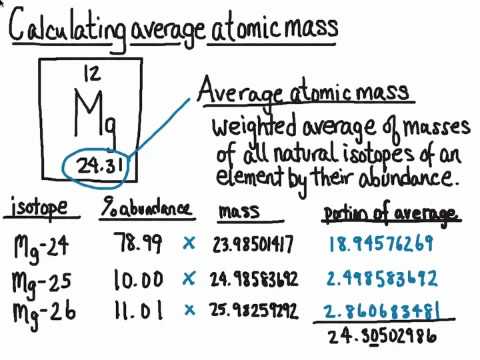
Atomic masses L.O.: Define the terms relative isotopic mass and relative atomic mass, based on the 12C scale; Calculate the relative atomic mass of. - ppt download